Electrochemical Etching

For those who’ve seen my DIY electrochemically etched Prody 99 head tube badge and want to know more about the process (maybe even have a go), this page contains the info you’ll need to understand what’s involved.
There are two relatively ‘safe’ (non-acidic) electrochemical etching methods that have been developed by printmakers (artists) for etching – one for warm coloured metals, one for cool. For etching brass and copper you’ll need ferric chloride (Google it if you want to know more about that process). For etching cool (silvery coloured) metals like aluminium, zinc or steel, you’ll need to create a saline sulphate solution – the method I used, and the method that’s detailed in the guide below. (NB. Never mix the two solutions.)
Here’s a little photo diary of my process…
My original Agent 99 artwork, drawn in black micro-liner from a photo of Barbara Feldon’s original 99. To work with the etching process (much like any process developed for printmaking) the aim was to simplify the features as much as possible, while keeping it iconically recognisable…

I scanned the drawing, traced it up as a vector in Adobe Illustrator, added my Ninety Nine logo, and the Prody and Colony logos, to create the final badge design that would be etched. I then reversed the design, and inverted the colours (black for the high points that would end up polished silver, white for the recesses that would be black), and laser printed it onto an acetate sheet ready to transfer to the aluminium plate…

On a lightly sanded sheet of 1mm aluminium sprayed with a little Isopropyl alcohol, I placed the acetate image print side down and used a hot iron to melt the toner ink and transfer the design to the aluminium plate (the iron was set to the minimum steam setting – no hotter as it can buckle the acetate and distort the design)…

I stacked up a bunch of transfers to experiment with – at this stage is all about trial and error, testing out the process to get familiar with its techniques and eccentricities…

Initial tests in the saline sulphate solution proved that while the acetate transfer method might work well for the light etch required for printmaking, it lacked the integrity for the deeper etch I needed. You can see the high points (silver areas) are lightly pitted and uneven…

Problem solving: I experimented with acrylic paint as the resist medium. I used Tamiya model acrylic that sets hard – as opposed to artist’s acrylics, they have too much elasticity and weaker adherence to non-porous surfaces. I scribbled with acrylic and a brush on the little test piece of aluminium below, and when it was dry I dropped it in the salt etch for 30mins. Stoked with the depth and edge integrity – streets ahead of using the acetate transfer alone…

Now the fiddly part… I used a brush with 3 bristles (yep, THREE! it had to be fine enough to handle the level of detail) to paint the acrylic resist medium on a freshly transferred plate. It took 2-3hrs and a steady hand to cover all the areas of transfer, taking great care to get all the fine details and render the edges smooth and straight…

Acrylic dried and ready to etch…

The colours that are produced in the saline sulphate etch are ridiculously rad. I found (after a few tests) that gently wiping the red waste off the plate with a plastic spoon every 5mins or so helps the speed and crispness of the etch. To watch a little time lapse Instagram video I took of the plate in the etch (complete with the excitement of hydrogen bubbles!), click here: http://instagram.com/p/vx42XgQzEt/ …

30mins in the salt etch, ready to clean the acrylic off with acetone and check the integrity of the details, edges and high points….

Super happy with the acrylic resist method! The time spent applying it is totally worth it. I etched a couple of different versions of the plate experimenting with solution strengths and etch times. The badge on the right was the winner – I used a slightly stronger (refreshed) solution for less time (30mins). As opposed to the badge on the left that was etched in an almost exhausted solution for about 50minutes and lost a lot of detail and edge quality….

Cutting out the badge by hand with my trusty piercing saw…

Cut out, burrs filed away from the edges, and ready for a spin on the polishing wheel before bending…

My DIY bending tool… creatively named ‘Bender’ (I know, I put a lot of thought into it… ;))… lined with felt to protect the surface of the badge…
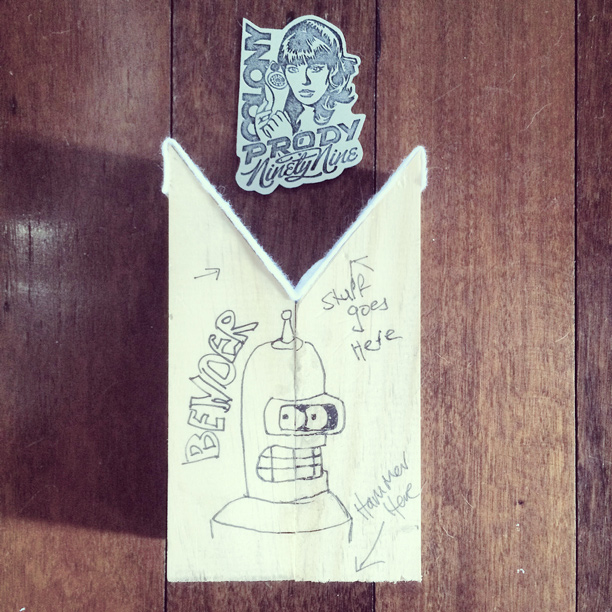
Curved to perfectly fit the Prody…

After a little extra black fill to enhance the contrast (but not so much as too lose the patina in the etched recesses), fitted and sitting pretty…

WANNA GIVE IT A GO?
This is the part where you choose between the red pill or the blue pill.
The red pill? Keep reading and dive headfirst down the rabbit hole! And it’s quite the rabbit hole…
The blue pill – chill and hit up your nearest industrial etching outfit and have them etch your design for you.
Things to consider before you start:
1. Hold your horses before heading down to your local hardware store or garden centre and purchase the nearest bag labeled ‘Copper Sulphate’. The stuff used for agricultural and horticultural applications is a blue crystalline form called PENTAHYDROUS copper sulphate (Cu2SO4), DON’T USE IT FOR ETCHING. Seriously; as cheap and tempting as it is to try, it’s not pure enough and often has weird additives that will give you erratic results. You need ANHYDROUS copper sulphate, a pure white powder (CuSO4). Anhydrous copper sulphate is a little harder to get, but eBay will hook you up if you get stuck. I get mine from Ireland, 250g at a time (you don’t need a lot to make small stuff like badges).
2. While this is a relatively safe method of electrochemical etching, this is still a CHEMICAL solution, and safety precautions must be taken every step of the way. It’s extremely easy to expose yourself to toxic levels of copper just by getting this solution on your skin. Make sure you wear gloves, mask and eye protection when mixing and working with the copper sulphate and saline sulphate solution. It’s an irritant, so if splash yourself you’ll feel an intense itch on your skin – stop what you’re doing and wash it off immediately.
3. Disposal: You absolutely must not pour this solution down the drain, or into the ground or waterways. Saline Sulphate etch is extremely toxic in the natural environment. It can and will destroy wild life and natural eco-systems. If you’re going to try this process it’s critical that you follow the correct disposal guidelines at the bottom of this page. Please, GIVE A SHIT about the environment – this isn’t even like pouring bleach down the drain (ahhh… noooo! Don’t do that either…! ) this is a MUCH BIGGER DEAL.
4. If you’ve read the 3 points above and are still keen to give it a shot, then note well that this is one substantial rabbit hole you’re about to dive down. Getting good results is not easy, and requires a lot of experimentation. This is a process designed for intaglio print making, which means it’s designed for a very light etch, just enough change in surface tension to grab small amount of printing ink and deliver it, under pressure, to damp paper. If you want a deep etch it’s a tough task to find a balance between depth and detail… the longer you leave it in, the deeper the etch will go, but you can start to lose your fine lines and details.
5. Zen. Find some. If you’re an impatient person, particularly one who’s trying to etch a detailed design, this could be a frustrating process. The acetate heat transfer resist method just doesn’t cut it for deep etching, so be prepared to wield a teeny tiny paintbrush for an inordinate period. Model acrylic works brilliantly as a resist medium and leaves your high points well protected. Just as a rough guide – it takes me 2-3 hours to hand paint resist medium over design as detailed as my Proddy 99 badge. 2-3 hours per badge. (I’ve wondered whether screen printing and heat sealing a design onto the plate would be a solution for repeats of the same design, but I’d need to experiment with how screen printing ink holds up as a resist medium.)
The Process
The article below is in lifted from http://www.nontoxicprint.com/etchzincsteelaluminum.htm – their guide and information is excellent, all credit goes to them. Please read ALL of the article below carefully before you buy your supplies and start the process.
Etching Zinc, Steel, Aluminum: The Saline Sulfate Etch
A new etching solution for zinc, steel and aluminum
By Friedhard Kiekeben (with special thanks to Cedric Green and Nik Semenoff for their vision and research)
Note of caution: all safer etching methods involve more or less hazardous chemicals and by-products, they are not ‘nontoxic’. Safe handling, informed training, and various protective measures are essential requirements in metal etching.
The silvery metals of zinc, mild steel, and aluminum offer many unique pictorial possibilities. Plates of these materials can be purchased both from printmaking suppliers and industrial sheet metal stockists. Whilst zinc is the most popular metal used by European etchers, this very expressive kind of plate is only just becoming more widely available in the USA where it can now be purchased as an interior decorating material. Both aluminum and mild steel are ubiquitous and cheap metals used in construction and industry, and both offer an interesting expressive repertoire when etched. Steel offers the added advantage of being very hard wearing, thus allowing for large edition sizes. The Saline Sulfate Etch works very well for straight tray etching of any of the silvery metals, and additional measures such as heating or aeration are not required. Zinc plates, especially, etch with great speed and ease. Often a deep etch is completed within a matter of minutes. A copper sulfate based etching solution was first suggested by two printmaking experts, NIK SEMENOFF (Salt Etch, Leonardo, 1998) and CEDRIC GREEN (The Bordeaux Etch), in response to health and environmental concerns. It is thought that Goya and his contemporaries used a similar method which unfortunately fell out of favor later on.
The invaluable groundwork and feedback of Green and Semenoff helped me eliminate early issues with the Edinburgh Etch (See ETCH COPPER AND BRASS), and paved the way towards the formulation of a comprehensive metal salt etching system which can now serve as a complete replacement for the acid etching approach. In developing the Saline Sulfate Etch I was aiming to present the most effective and practical solution for artists and educators who want to etch zinc and the other silver-grey metals easily and safely. I believe that the new Saline Sulfate etching method provides an ideal substitute for the much more toxic nitric acid etch for these metals. Feedback from scientists and artists alike confirms this and the resulting prints speak for themselves.
A saturated copper sulfate solution makes a good mordant for zinc, but due to lack of a catalyst, etching is somewhat slow and the solution becomes exhausted quickly. Similar to my thinking behind the Edinburgh Etch, I looked into ways in which the electrolytic eroding potential of copper sulfate could be harnessed more fully. I reasoned that, as with ferric, the chemical ‘hydrolysis bond’ formed between the metal salt and water might account for a loss of reactivity. Tests showed that a solution made up from equal parts of copper sulfate and sodium chloride (i.e. cooking salt) activates the etch by diminishing the bond with water. The Saline Sulfate Etch for etching zinc is about three times more active than a straight copper sulfate solution without salt; it also produces a very crisp etch. During biting a coppery sediment of metal hydroxides and oxides floats to the surface, thus keeping the bitten work from clogging up. Etching can also be aided by occasionally brushing the plate surface with a soft brush; delicate marks, such as a spray aquatint or soft ground should, however, be etched without brushing. The solution works more effectively if floating solids are regularly skimmed off with a brush or strainer and removed from the bath, this keeps the solution from turning alkaline and extends its usable life.
Making up the Saline Sulfate Etch
To etch silvery-grey metals: zinc, mild steel and aluminum.
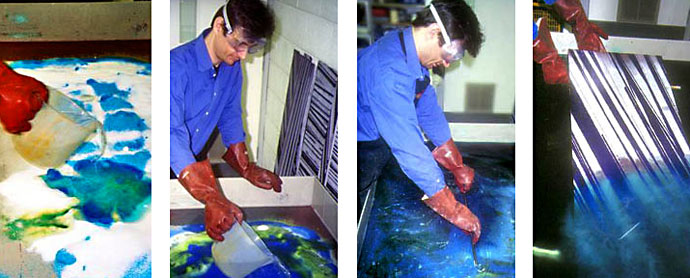
Dissolving salt and copper sulfate in hot water. Agitation with squeegees or brushes helps to fully dissolve the solid salt particles. Only dissolved salts provide metal ions and an electric charge crucial for etching. Best wear goggles and a dust mask when handling copper sulfate.
The solution is made up from equal amounts of copper sulfate and cooking salt which readily dissolve in warm water. It is recommended to use anhydrous copper sulfate which is supplied at a reasonable price in larger quantities by chemical wholesalers. Do not use agricultural supplies as these may contain additives. As with most etching chemicals, specifically ask for technical grade or industrial grade copper sulfate. Laboratory grade chemicals are much more expensive and are less suitable for etching.
MATERIALS
Products and equipment needed to make up the Saline Sulfate Etch:
- anhydrous copper sulfate
- cooking salt
- supply of hot water
- heavy-duty gloves
- safety goggles
- dust mask
- wooden stick or stiff plastic brush for mixing
- bucket
- etching tray
METHOD
Make up the Saline Sulfate Etch as follows:
Put on gloves, dust mask, and safety goggles when handling the crystals to avoid touching or inhaling dust particles.
Carefully place equal amounts of copper sulfate (e.g.100g*) and cooking salt (e.g. 100g*) into a bucket or straight into a small etching tray.
It is useful but not essential to premix the dry powders before diluting with water.
Add 500ml* of hot water and stir all the ingredients together with a wooden stick or a stiff brush.
The solution turns into a green sludge.
Add another 500ml to 1liter* of warm water whilst stirring continuously.
Most of the crystals should dissolve within 5 to 10 minutes of mixing, producing a dark green liquid.
SAMPLE QUANTITIES
SMALL PROJECT:
100g copper sulfate + 100g sodium chloride
added to 500ml hot water
added to 500ml to 1 liter warm water
LARGE PROJECT:
1kg copper sulfate + 1kg sodium chloride
added to 5 liters hot water
added to 5 litres warm water
*The above ratios/quantities should suffice for a small etching project, using a small tray. For bigger projects simply increase the quantities accordingly.
In a busy print studio it is advisable to make up a substantial quantity of Saline Sulfate Etch in a large tray or tank. This may contain even several gallons of solution. The increase in electric charge makes a large volume of metal salt solution longer-lived than a small one. The kind of high-sided plastic trays with lids sold for under bed storage make ideal metal salt etching trays. Acid unit manufacturers and other plastics prefabrication companies can manufacture professional trays from welded polypropylene to specification. A deep tray is best fitted with a slatted plastic or wooden grid to elevate plates above the crystalline deposit that builds up in the bath, and to help when removing plates from the bath. The Saline Sulfate Etch can be used as a universal etching solution for all three metals: zinc, mild steel, and aluminum.
TIP: Using and Purchasing Copper Sulfate
Safe to Use:
Use it Safely! Remember, Copper Sulfate is still a chemical and toxic to humans if ingested, so make sure you follow safety instructions and read MSDS sheets prior to use. Always wear gloves, goggles and a mask when handling any etching chemicals. Do not touch, inhale or ingest.
Safe to Ship
For the printmaker who wants to use the Saline Sulfate Etch, getting hold of small (rather than industrial) quantities of Copper Sulfate is easy. It can be ordered over the internet and because it is a low hazard material, the delivery came by ordinary mail services – simple, safe and economical too. No need for the specialist carriers required when shipping traditional printmaking mordants.
Etching Aluminum
Spelling Note – US: Aluminum, UK/AUS/NZ: Aluminium

Vincent Finazzo, Delineation I, aluminum etching, 2008
Due to its softness and its coarse atomic structure, aluminum is somewhat less well suited for the entire spectrum of intaglio printmaking than other kinds of metal. However, since plates are cheaply available from sheet metal merchants (you may even get them for free from commercial printers) many printmakers use them for dypoint or as a substrate for photopolymer work. Previously, this lightweight metal was rarely used for intaglio etching; now, by using the Saline Sulfate Etch, it provides unique benefits and qualities.
In etching, aquatint is normally used to fill open areas on the plate with durable tones or a black. The Saline Sulfate Etch for aluminum is self-aquatinting. During etching a very distinctive and durable surface roughness occurs in the open areas; this crystalline texture can produce a beautiful black on the print all by itself. As a result, there is no such thing as open bite in this process because all etched areas become carriers for etching ink, thus enhancing the graphic potential of the process.
Unusually, neither of the basic components of the Saline Sulfate Etch, i.e. copper sulfate and salt, have any corrosive effect on the metal by themselves. Etching becomes possible when both substances act on the metal in combination. While all other metals easily erode as long as they are grease free, the surface of aluminum plates is best treated with fine wire wool to make the surface more susceptible to the etching process. This should be done before any acrylic grounds are applied to the plate.
As with zinc, the Saline Sulfate Etch for aluminum produces of a loose coppery sediment which floats to the surface and needs to be removed regularly. Unlike with zinc, a continuous rising of small hydrogen bubbles (not considered a hazard) also indicates that etching is in progress.
Tip: When stripping acrylics off an etched aluminum plate, ensure that plates are not left in the soda ash stripping solution too long as this will etch the plate further.
Etching Steel

Vincent Finazzo, Delineation III, steel etching, 2008
Steel plates need to be purchased as cold rolled mild steel; tempered or hot rolled varieties of steel plate are not suitable for etching. Plates usually have a coating of grease which needs to be removed before any creative work with resists can commence.
When etching steel, use the same recipe for the Saline Sulfate Etch as given above.
Once a plate has been etched to the required depth and rinsed it is crucial to blot off any remaining dampness with paper towels and then speed dry plates otherwise the steel surface will quickly rust.
The Magic of Electro-Chemical Etching
Metal Salt etching is a new methodology which is fundamentally different from the aggressive chemistry of traditional acid etching with its hazardous gas emissions and by-products. The new method is akin to creating a liquid battery. The etching battery consists of two types of metal that act as the charged poles of the battery – anode and cathode – and an electrolyte medium (here the salt solution) that transmits electric charge and allows metal ions to migrate. The process lacks none of the mystique of acid etching. The alchemical magic of seeing a solid metal vanish in a liquid is in many ways an even more tactile and engaging activity and produces startlingly good results.
The dissolved copper ions (from copper sulfate) create a powerful electric potential which literally pulls away the surface atoms of the silver-grey metal plates it comes into contact with. The more copper ions there are contained in the solution the greater the etching potential of the bath; therefore this process works best when used in a larger tray or tank which has a greater electric charge and can etch more metal for longer. The etching process produces various solid by-products; the metal oxides and hydroxides of the etched plate on the one hand, and a fine deposit of pure atomic copper on the other.
While a Saline Sulfate bath is active and usable it retains a green colour, although after a while the original translucent green is replaced by what looks like a much more unattractive soup of green sludge. But worry not: the sludgy looking bath etches just as well as a translucent one. Stir up and mix this sludge with a stick or a brush before each etching session. This re-dissolves the copper compounds that are crucial to building up electric charge for further etching. Once the etching capacity of the bath does slow down, there are two options: Refreshing the solution or Recycling, Neutralisation and Disposal.
Refreshing a Saline Sulfate Solution
In previous publications I advocated recycling the Saline Sulfate solution once spent, but recently I have found a way to significantly extend its usable life. Simply make up a further batch of a copper sulfate or sodium chloride mixture in a bucket and add just enough hot water to dissolve. Add this refreshing mixture to the etching tank; stir, and the bath will be reactivated. This procedure can be repeated three or four times, and the usable life of the bath can thus be extended from several weeks to up to half a year. As time goes on more solids will build up in this long-lasting bath and can eventually affect the cleanness of the etch. Etching plates on a slatted grid that elevates them above the salt deposits can remedy this unwanted effect.
I have also observed an effect, first described by Nik Semenoff, when a seemingly spent copper sulfate based etching solution self-regenerates; this is indicated by the return of the green coloring to a solution that has been left unattended for several weeks (regular stirring and the addition of hot water aids this process). The solution has regained dissolved copper ions and an electric charge, and can be used for etching once again.
Recycling, Neutralisation and Disposal
The process comes full circle. The very action that makes the Saline Sulfate Etch work so wonderfully as an etching bath – the depletion of copper ions – also facilitates its recycling. Concentrated copper ions are regarded as an aquatic pollutant and must not be allowed to get into waste water. As more and more copper ions react with the metal plate during etching these are converted into their inert cousins: solid copper atoms. If a sufficient quantity of metal is etched, eventually all copper ions are removed. A fully depleted bath is recognizable by two features: (i) the solution no longer corrodes metal and (ii) the solution is no longer green, it is clear.

Spent solution easily separates into a clear liquid and solid particles.
IMPORTANT:
Environmental Safety Although copper sulfate is a comparatively safe chemical for etching, it is considered a marine pollutant, and if present in rivers or lakes it can kill fish. It is crucial that solutions containing this salt are never poured down a drain without following the above instructions. Only a spent solution that is lacking the green coloration (which indicates the presence of copper ions) and that has been neutralized with sodium carbonate is safe to be discarded.
If you are in the USA, the Small Business section of your local Environmental Protection Agency offers free advice on the safe disposal of exhausted metal salts and liquid ferric-based etching solutions. Solid zinc, aluminum, steel and copper residues may be safe for local disposal once any copper sulfate content has been fully removed. Professional printmaking studios should utilize a commercial chemical disposal firm to pick up spent etching by-products. Visit your local EPA page for details of local firms. Visit www.epa.gov.
RECYLE, NEUTRALISATION AND DISPOSAL METHOD
Prepare a spent etching bath for recycling as follows:
- Add hot water to the bath to re-dissolve any solid sulfate particles and stir.
- Add a pile of metal off-cuts – zinc, steel or aluminum – to the tray
- Leave to act overnight.
- On the following day, drain off the liquid into a bucket and add sodium carbonate (about two or three cups per bucket).
- Once fizzing stops the liquid can be discarded.
- The remaining solids can now be left to dry out. Keep in labeled, sealed containers and then treat as dry waste.
_________
A Guide to the Safe Use of the Metal Salt Etching System
Prior to use, read the safety instructions given on all etching chemicals and in their MSDS sheets.
Follow instructions carefully.
Do not touch, inhale or ingest etching chemicals.
Always wear strong gloves, eye protection, a dust mask and long apron as advised.
The Metal Salt Etching system is safe to use if the warm and the cold colored metals are etched in their respective warm and cold colored solutions.
Use the rust/orange colored Edinburgh Etch (ferric based) for etching the reddish metals: copper or brass. Use the blue/green colored Saline Sulfate Etch (copper sulfate based) for etching the silvery metals: mild steel, zinc, and aluminum. DO NOT use the Edinburgh Etch or a ferric bath to etch zinc or aluminum, as this may cause a hazardous chemical reaction.
Also read and follow etching precautions given at The New Etching Chemistry page
NOTE: Although considerably safer, the new systems still utilize harmful corrosive chemicals and their reactive properties, and it is very important to follow all safety instructions in their use. Medical science suggests that as an extra precaution women should not engage in extensive etching practice of any kind (acid or salt based) during pregnancy to safeguard against the possibility of reproductive damage.
